𝗨𝗻𝗱𝗲𝗿𝘀𝘁𝗮𝗻𝗱𝗶𝗻𝗴 “𝗠+𝟭𝟲” 𝗜𝗺𝗽𝘂𝗿𝗶𝘁𝗶𝗲𝘀 𝗶𝗻 𝗣𝗲𝗽𝘁𝗶𝗱𝗲 𝗦𝘆𝗻𝘁𝗵𝗲𝘀𝗶𝘀
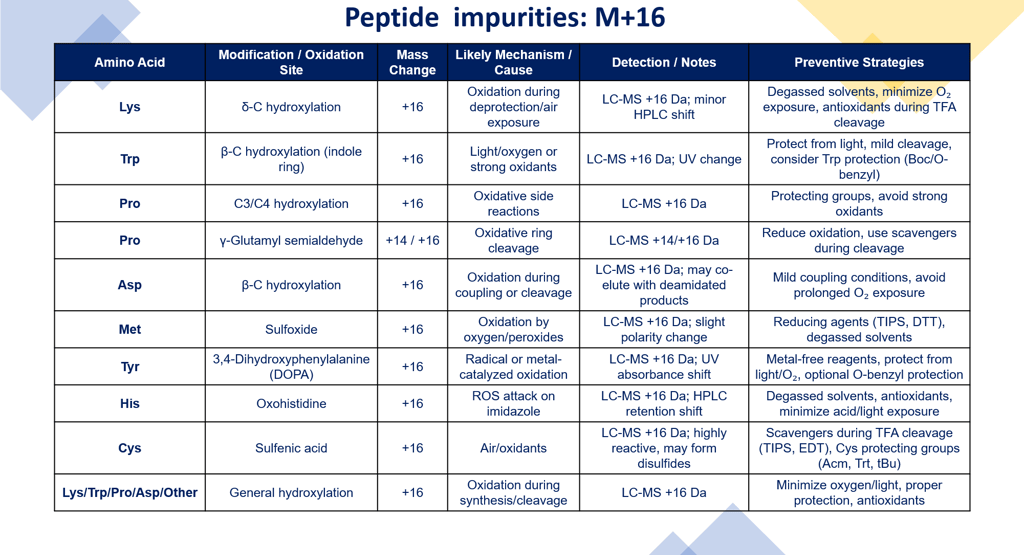

1) Lysine (δ-carbon hydroxylation)
Position affected: δ-C of the side chain (ε-amino group is on δ-C).
Mechanism: Often occurs under strong oxidizing conditions, e.g., during Fmoc deprotection (piperidine) in the presence of oxygen, or during TFA cleavage if traces of oxidants are present.
Impact: Adds +16 Da (OH) to the Lys residue.
Detection: LC-MS shows mass increase of +16 Da; may slightly change retention time in HPLC due to increased polarity.
Preventive measures:
Use fresh, degassed solvents.
Minimize air exposure during cleavage and handling.
Include antioxidants (e.g., TIPS during TFA cleavage).
2) Tryptophan (β-carbon hydroxylation)
Position affected: β-C of indole ring.
Mechanism: Indole is highly sensitive to oxidation. Hydroxylation usually occurs during light exposure, oxygen, or strong oxidants.
Impact: +16 Da; can lead to trp side-chain modifications like oxindole formation.
Detection: LC-MS; UV absorbance of Trp changes due to indole modification.
Preventive measures:
Protect from light.
Use mild cleavage conditions; avoid strong acids or oxidizing reagents if possible.
Consider Trp protection (Boc, O-benzyl) if long exposure is needed.
3) Proline (C3 or C4 hydroxylation)
Position affected: γ (C3) or δ (C4) carbon of pyrrolidine ring.
Mechanism: Often enzymatic in nature in vivo, but in SPPS, can arise from strong oxidative conditions during cleavage or resin treatment, especially if side-chain protection is absent.
Impact: +16 Da per hydroxylation.
Detection: LC-MS; may show altered retention due to hydrogen bonding; sometimes hard to separate.
Preventive measures:
Use appropriate protecting groups (Boc or tBu on adjacent residues).
Avoid overexposure to oxidizing conditions during TFA cleavage.
4) Aspartate (β-carbon hydroxylation)
Position affected: β-C (side-chain carboxyl).
Mechanism: Usually occurs via oxidative attack under acidic or radical conditions, sometimes during activation of Asp for coupling. Racemization can accompany oxidation.
Impact: +16 Da; may lead to Asp–Asp or Asp–X side products if β-hydroxylation alters reactivity.
Detection: LC-MS and HPLC; may co-elute with deamidated products, careful mass spec analysis needed.
Preventive measures:
Use mild coupling conditions, e.g., HBTU/HATU with minimal base excess.
Avoid prolonged exposure to oxygen or radical-forming reagents.
5) Methionine → Methionine Sulfoxide
Reaction: Oxidation of the sulfur atom in the thioether side chain.
Mass change: +16 Da per Met residue.
Mechanism: Oxidation by oxygen, peroxides, or trace oxidants during SPPS or TFA cleavage.
Detection: LC-MS: +16 Da; sometimes HPLC shows slight shift due to increased polarity.
Impact: Can affect peptide activity, solubility, or folding.
Prevention:
Use reducing agents (TIPS, DTT) during TFA cleavage.
Minimize air/oxygen exposure.
Store peptides at low temperature under inert atmosphere.
6) Tyrosine → 3,4-Dihydroxyphenylalanine (DOPA)
Reaction: Hydroxylation of phenolic ring at positions 3 and 4.
Mass change: +16 Da per hydroxylation.
Mechanism: Radical or metal-catalyzed oxidation (Cu²⁺, Fe³⁺) during synthesis or storage.
Detection: LC-MS +16 Da; UV absorbance shifts due to catechol formation.
Impact: Alters polarity and reactivity; can lead to cross-linking (quinone formation).
Prevention:
Avoid metal contamination in solvents/reagents.
Protect from light and oxygen.
Consider temporary protecting groups (e.g., O-benzyl) if long synthesis.
7) Histidine → Oxohistidine
Reaction: Oxidation of the imidazole ring, forming keto-imidazole derivatives.
Mass change: +16 Da per His oxidation.
Mechanism: Reactive oxygen species attack imidazole, often during TFA cleavage or storage.
Detection: LC-MS: +16 Da; may change HPLC retention.
Impact: Can interfere with metal binding or peptide activity.
Prevention:
Degassed solvents and antioxidants.
Minimize prolonged acid exposure or light.
8) Cysteine → Sulfenic Acid (Cys-SOH)
Reaction: Oxidation of thiol to sulfenic acid (intermediate before sulfinic/sulfonic acids).
Mass change: +16 Da.
Mechanism: Air oxidation, peroxides, or during TFA cleavage if no scavenger.
Detection: LC-MS: +16 Da; highly reactive and may form disulfides or sulfonic acids.
Impact: Can cause disulfide scrambling or peptide aggregation.
Prevention:
Use scavengers during TFA cleavage (TIPS, EDT, thioanisole).
Protect Cys with Acm, Trt, or tBu groups.
Minimize exposure to oxygen.
9) Proline → γ-Glutamyl Semialdehyde (oxidized proline)
Reaction: Oxidation of the pyrrolidine ring to form an aldehyde at γ-C.
Mass change: +14 Da (because of loss of two hydrogens and addition of oxygen, sometimes represented as +16 depending on tautomer).
Mechanism: Can occur during TFA cleavage under oxidative conditions or prolonged air exposure.
Detection: LC-MS: +14/+16 Da; retention time changes.
Impact: Can lead to peptide backbone cross-linking (Schiff base formation).
Prevention:
Minimize exposure to oxidizing conditions.
Include reducing scavengers during cleavage.
Avoid metals that catalyze oxidation.
General Notes on Hydroxylation in Peptide Synthesis
1. Hydroxylation always adds +16 Da per site, so MS analysis is straightforward.
2. More common in residues with electron-rich side chains (Trp, Lys, Pro).
3. Often occurs post-cleavage or during prolonged reaction times, not just on resin.
4. Minimizing oxygen, light, and strong oxidizing agents is the best preventive strategy.
