Synthesis of Radiolabel peptides
12/31/20253 min read
1. Peptide Assembly (Fmoc-SPPS)
Resin: Rink amide–MBHA.
Synthesis: Standard Fmoc cycles
Manual coupling (large scale) or
Biotage Syro II automated synthesizer (small scale).
Complete linear peptide sequence on resin.
2. Cleavage & Crude Peptide Preparation
Cleavage: TFA-based cocktail.
Precipitate in cold isopropyl ether.
Centrifuge (3 min, 3000 rpm).
Wash solid with isopropyl ether (×2).
Dry under vacuum (2 h).
Dissolve crude peptide in MeCN/H₂O (40:60, v/v).
3. TATA Bicyclization (Solution Phase)
Peptide concentration: ~1 mM.
Add TATA scaffold (1.0 eq).
Adjust to pH 8.0 using 1.0 M NH₄HCO₃ (aq.).
Stir at RT.
Monitor cyclization by LC–MS.
4. Quench, Purification & Characterization
Quench with cysteine (10 eq vs peptide).
Adjust pH to 7.0 (1 M HCl).
Lyophilize.
Purify by RP-HPLC.
Pool fractions with correct MW and purity.
Confirm identity by MALDI-TOF + HPLC or LC–MS.
Lyophilize purified bicyclic peptide.
Radiolabeling of Peptides
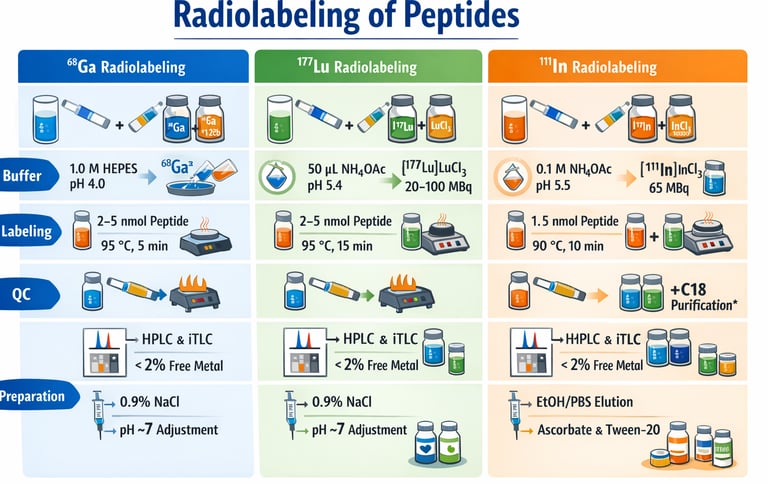
A. ⁶⁸Ga Radiolabeling
Buffering:
Mix HEPES buffer (1.0 M, pH 4.03) + ⁶⁸Ga-eluate (30–120 MBq) in equal volumes (40–150 µL).
Adjust pH to 4.0 if needed (10% NaOH).
Labeling:
Add precursor: 2–5 nmol (1 mM in DMSO).
Heat 95 °C, 5 min.
QC & Use:
Analyze by HPLC + iTLC.
Use directly if free/colloidal ⁶⁸Ga < 2%.
Dilute with 0.9% NaCl, adjust to pH ~7 (30% NaOH).
B. ¹⁷⁷Lu Radiolabeling
Reaction Setup:
NH₄OAc buffer (50 µL, pH 5.4).
Precursor: 2–5 nmol (1 mM in DMSO).
Add [¹⁷⁷Lu]LuCl₃ (20–100 MBq) in 0.04 M HCl.
Labeling:
Heat 95 °C, 15 min.
QC & Use:
Analyze by HPLC + iTLC.
Use without purification.
Dilute with 0.9% NaCl, adjust to pH ~7.
C. ¹¹¹In Radiolabeling
Reaction Setup:
NH₄OAc buffer (0.1 M, pH 5.5, 144 µL).
Precursor: 1.5 nmol (0.2 mM in DMSO).
Add [¹¹¹In]InCl₃ (65 MBq).
Labeling:
Heat 90 °C, 10 min, shake 500 rpm.
QC & Purification:
Analyze by HPLC + iTLC.
Purify using C18 Sep-Pak (EtOH/PBS elution).
Formulation:
Final volume: 1 mL PBS.
Add 5 mg sodium ascorbate + 0.05% Tween-20.
Practical Notes
pH control is critical:
⁶⁸Ga: pH 3.5–4.5 (optimal chelation).
¹⁷⁷Lu / ¹¹¹In: pH 5.0–5.5.
Short heating times minimize radiolysis and peptide degradation.
Sodium ascorbate effectively suppresses radiolysis in ¹¹¹In products.
Avoid excess DMSO (>10%) to prevent reduced labeling efficiency.
Troubleshooting
Low radiochemical yield:
Check buffer pH and metal contamination.
Increase precursor amount slightly (up to 10 nmol).
High colloidal metal fraction:
Reduce heating time or metal activity.
Improve buffer purity and pH accuracy.
Poor in vivo stability:
Add antioxidants (ascorbate, methionine).
Minimize delay between labelling and injection.
Radiation Safety Measures
All radiolabelling performed in designated hot cells or shielded fume hoods.
Wear dosimeter, lab coat, double gloves, and eye protection.
Use lead shielding for ⁶⁸Ga and tungsten shielding for ¹⁷⁷Lu/¹¹¹In.
Monitor contamination regularly using GM counter or dose calibrator.
Dispose radioactive waste according to institutional and regulatory guidelines.
HPLC and Radio-HPLC Analyses
Analytical HPLC
HPLC analyses were performed using two methods:
Method A:
Agilent 1100 series HPLC (Agilent Technologies) equipped with a Chromolith RP-18e column (100 × 4.6 mm, 2 µm, 130 Å; Merck).Method B:
Agilent 1290 UPLC (Agilent Technologies) equipped with an ACQUITY UPLC CSH C18 column (150 × 2.1 mm, 1.7 µm, 130 Å).
UV detection was performed at 220 nm and/or 254 nm.
Radio-HPLC
Radio-HPLC analyses for ⁶⁸Ga- and ¹⁷⁷Lu-labeled peptides were conducted on an Agilent 1100 series HPLC system equipped with a RAMONA Star radiodetector (Elysia Raytest).
For ¹¹¹In-labeled peptides, radio-HPLC was performed using an Agilent 1100 series HPLC equipped with a Jupiter C18 column (150 × 2.6 mm, 5 µm, 300 Å; Phenomenex) and a Raytest Gabi radiodetector (Elysia Raytest).
In all cases, separations were carried out using linear gradients with the following mobile phases:
Solvent A: 0.1% TFA in Milli-Q H₂O
Solvent B: 0.1% TFA, 10% Milli-Q H₂O in MeCN
Flow rates ranged from 0.4–1.0 mL/min, depending on column and method.
Radio-TLC Analyses
Radio-TLC analyses for ⁶⁸Ga- and ¹⁷⁷Lu-labeled peptides were performed on iTLC-SG glass microfiber paper (Agilent) using 0.5 M NH₄OAc(aq.):DMF (1:1, v/v) as the mobile phase.
For ¹¹¹In-labeled peptides, iTLC was developed using 0.1 mM NH₄OAc(aq.) containing 25 nM EDTA.
TLC plates were analyzed for 5 min using either a miniGITA Star (Elysia Raytest) or a Cyclone Plus phosphor imager (PerkinElmer).
Reference:
Sharma, A.K., Gupta, K., Mishra, A., Lofland, G., Chen, S.Y., Marsh, I., Fair, P.T., Hobbs, R.F., Armstrong, T.M., Jaffee, E.M., Gabrielson, E.W., Zheng, L., Nimmagadda, S. (2025). EphA2-targeted alpha-particle theranostics for enhancing PDAC treatment. Theranostics, 15(10), 4229-4246. https://doi.org/10.7150/thno.106948
