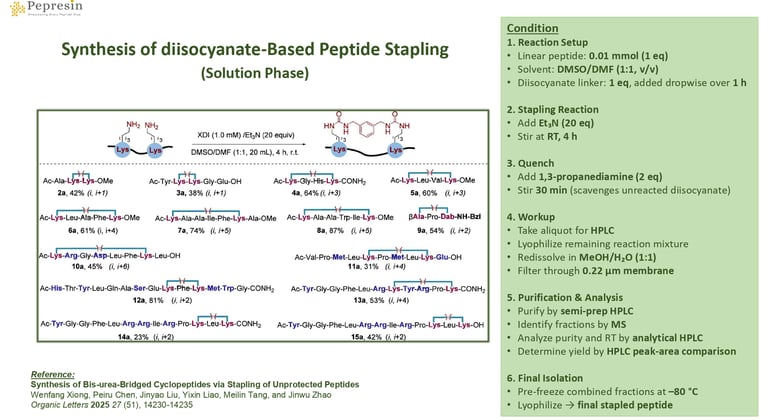
Synthesis of diisocyanate-Based Peptide Stapling (Solution Phase)
1. Preparation
1.1. Place a magnetic stir bar in a 25 mL round-bottom flask.
1.2. Dissolve the linear peptide (0.01 mmol, 1.0 equiv.) in DMSO/DMF (1:1 v/v, 10 mL).
1.3. Prepare a separate solution of diisocyanate (1.0 equiv.) in DMSO/DMF (1:1 v/v, 10 mL).
2. Reaction Setup
2.1. Add the diisocyanate solution dropwise to the peptide solution over 1 h using a constant-pressure dropping funnel.
2.2. Add Et₃N (20 equiv.) to the reaction mixture.
2.3. Stir at room temperature for 4 h.
3. Quenching
3.1. Add 1,3-propanediamine (2.0 equiv.) to quench and capture mono-reacted diisocyanate.
3.2. Stir for 30 min.
4. Crude Sample Preparation
4.1. Withdraw a small aliquot for analytical HPLC.
4.2. Lyophilize the remaining reaction mixture to obtain crude solid.
5. Purification
5.1. Dissolve the crude solid in MeOH/H₂O (1:1 v/v, 4 mL).
5.2. Filter through a 0.22 μm membrane.
5.3. Purify the filtrate by semi-preparative HPLC.
5.4. Identify desired fractions by MS and combine.
6. Analysis & Yield Determination
6.1. Analyze the pooled fractions using analytical HPLC to confirm retention time.
6.2. Determine yield by comparing the product peak area with that of the starting reaction mixture.
7. Final Product
7.1. Pre-freeze the combined acetonitrile/water product solution at –80 °C.
7.2. Lyophilize to obtain the final stapled peptide.
Troubleshooting Guide for Diisocyanate-Mediated Peptide Stapling
1. Reaction Issues
Problem: Low conversion or incomplete stapling
Possible causes & fixes:
Peptide solubility issues
→ Pre-sonicate or warm slightly (≤35 °C) to fully dissolve.
→ Increase DMSO content up to 70% if necessary.
Steric hindrance near Lys side chains or N-termini
→ Increase diisocyanate to 1.2–1.5 equiv.
→ Extend reaction time to 6 h.
Insufficient base (Et₃N)
→ Ensure fresh Et₃N; moisture deactivates isocyanates.
→ Increase to 25–30 equiv. for difficult sequences.
Problem: Formation of mono-adducts (one end reacts, other remains unreacted)
Possible causes & fixes:
Fast consumption of one isocyanate end
→ Maintain slow, controlled dropwise addition (≥1 h).
→ Ensure reaction mixture is well-stirred and dilute.
Diisocyanate impurities or partial hydrolysis
→ Use freshly opened diisocyanate.
→ Avoid moisture; use dried syringes and glassware.
Problem: Excess polymerization or crosslinking
Possible causes & fixes:
High concentration
→ Keep final reaction concentration ≤0.5–1 mM peptide.
Moisture contamination leading to carbamate formation
→ Dry solvents thoroughly.
→ Perform reaction under N₂ if humidity is high.
Problem: Difficult purification or multiple side products
Fixes:
· Add scavenger (1,3-propanediamine) precisely at 2 equiv.
· Immediately quench after reaction completion to minimize side-reactions.
· For highly hydrophobic staples, increase MeOH in re-dissolution step.
2. Workup & Purification Issues
Problem: Low recovery after lyophilization
· Freeze the solution at –80 °C for ≥2 h before lyophilization.
· Avoid over-acidification or base in final solution—adjust pH to ~7.
Problem: Filtration loss
· Pre-wet the 0.22 μm filter with MeOH or MeCN.
· For sticky peptides, use PVDF or PTFE filters instead of nylon.
3. Safety & Risk Notes (Isocyanate Chemistry)
General Precautions
· Conduct all steps involving diisocyanates inside a certified fume hood.
· Wear nitrile gloves, lab coat, and safety glasses.
· Avoid any contact with moisture—diisocyanates react with water to form CO₂ and ureas.
Chemical Hazard Notes
Diisocyanates
· Strong respiratory sensitizers.
· Can cause severe skin and eye irritation.
· Must be handled with dry glassware and strict ventilation.
Triethylamine
· Flammable and corrosive.
· Use with adequate ventilation; avoid sparks/open flames.
DMSO & DMF
· Skin penetrants—avoid direct contact.
· Use gloves and minimize exposure.
Waste Disposal
· Dispose of diisocyanate-containing waste in segregated containers.
· Neutralize isocyanate waste with dilute amine solution before disposal (as per institutional guidelines).
· Follow local regulatory requirements for solvent and amine disposal.
Reference:
Synthesis of Bis-urea-Bridged Cyclopeptides via Stapling of Unprotected Peptides
Wenfang Xiong, Peiru Chen, Jinyao Liu, Yixin Liao, Meilin Tang, and Jinwu Zhao
Organic Letters 2025 27 (51), 14230-14235