Synthesis of Arginine N-Glycosylation Stapled Peptides
12/31/20253 min read
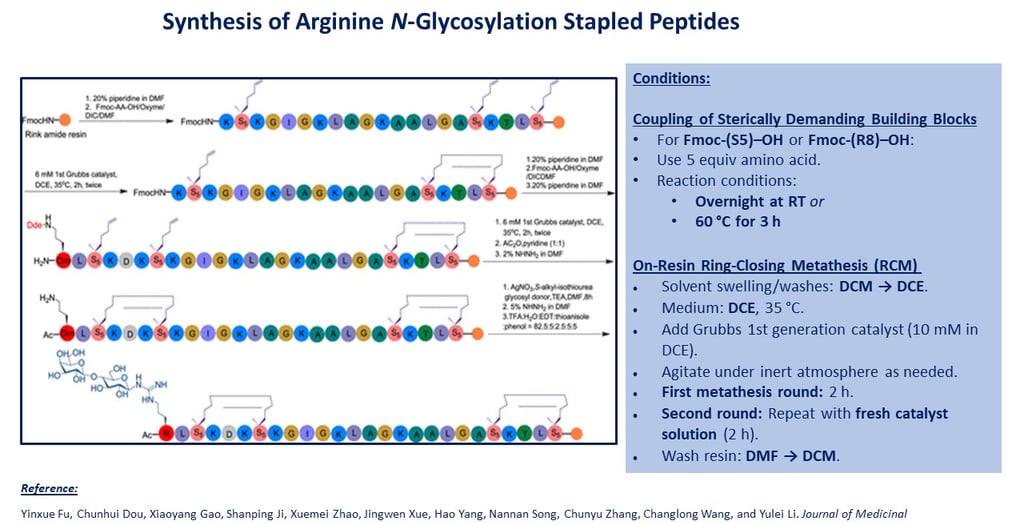
Resin and Loading
Rink amide MBHA resin (0.38 mmol/g) was used as the solid support.
Resin was pre-swollen in DMF prior to synthesis (typically 30 min).
Stepwise Protocol
1. Fmoc Deprotection
Treat resin with 20% piperidine in DMF (7 mL).
Shake at RT for 2 × 10 min or single 20 min treatment (laboratory dependent).
Wash resin with DMF (5 × resin volume).
Annotation: Removes the N-terminal Fmoc protecting group to expose the free amine for the next coupling step.
2. Standard Amino Acid Coupling
To the deprotected resin, add:
Fmoc-AA–OH (5 equiv)
Oxyma (5 equiv)
DIC (10 equiv)
DMF (7 mL total reaction volume)
Reaction:
Shake at 60 °C for 30 min.
Wash resin with DMF (3–5×).
Annotation: DIC/Oxyma activation ensures high coupling efficiency while minimizing racemization.
3. Coupling of Sterically Demanding Building Blocks
For Fmoc-(S5)–OH or Fmoc-(R8)–OH:
Use 5 equiv amino acid.
Reaction conditions:
Overnight at RT or 60 °C for 3 h
Annotation: Bulky or constrained amino acids require extended coupling to drive completeness.
4. On-Resin Ring-Closing Metathesis (RCM)
Solvent swelling/washes: DCM → DCE.
Medium: DCE, 35 °C.
Add Grubbs 1st generation catalyst (10 mM in DCE).
Agitate under inert atmosphere as needed.
First metathesis round: 2 h.
Second round: Repeat with fresh catalyst solution (2 h).
Wash resin: DMF → DCM.
Annotation: Performs macrocyclization or helix-stabilizing stapling through olefin metathesis.
5. Arginine N-Glycosylation (On-Resin)
To resin with exposed Arg side-chain amine, add:
Glycosyl donor (2 equiv)
AgNO₃ (2 equiv)
TEA (10 equiv)
DMF as solvent
Shake under exclusion of light until complete (time as per original method).
Annotation: AgNO₃ promotes formation of the reactive glycosyl donor complex enabling selective Arg glycosylation.
6. N-Terminal Acetylation
Add acetylation cocktail:
DIPEA : Ac₂O : DMF = 1 : 1 : 8 (v/v/v)
Shake at RT 10–30 min.
Annotation: Caps the N-terminus to prevent unwanted reactions and mimics native acetylated peptides.
7. Final Cleavage and Global Deprotection
Treat resin with cleavage cocktail:
H₂O / EDT / thioanisole / phenol / TFA = 5 : 2.5 : 5 : 5 : 82.5 (v/v/v/v/v)
Reaction time: 2–3 h at RT with gentle agitation.
Workup:
Concentrate filtrate.
Precipitate peptide using cold Et₂O.
Collect peptide by centrifugation.
Dissolve in MeCN/H₂O and lyophilize.
Purify via RP-HPLC.
Annotation: This cocktail provides strong acidolysis while efficiently scavenging cations and preventing side-chain damage (particularly for Trp, Met, Cys, and glycosylated residues).
Troubleshooting
Incomplete coupling: Increase temperature; double-couple; extend reaction.
Difficult residues (S5/R8): Use longer coupling or add HATU/OxymaPure for activation.
Darkening during AgNO₃ step: Insufficient light protection—wrap vessel in foil.
Low RCM yield: Ensure solvent dryness; add fresh catalyst; perform under N₂/Ar.
Impurities after TFA cleavage: Increase scavengers (phenol, EDT) or shorten cleavage time.
Analytical and Purification Methods
ESI-Q-TOF-MS Analysis
Electrospray ionization quadrupole time-of-flight mass spectrometry (ESI-Q-TOF-MS) was performed on a SCIEX X500R Accurate Mass Q-TOF LC/MS instrument. Samples were analyzed using standard positive-ion mode peptide acquisition parameters.
Analytical RP-HPLC Conditions
Analytical HPLC was performed on a SHIMADZU LC-20AD system. Chromatographic separation was carried out using a Welch C18 analytical column (4.6 × 250 mm, 5 μm) at a flow rate of 1.0 mL/min.
Detection wavelengths: 214 nm and 254 nm.
Solvent systems:
Buffer A: acetonitrile + 0.1% TFA
Buffer B: water + 0.1% TFA
Gradient: Linear increase of Buffer A from 10% → 90% over 20 min.
Preparative RP-HPLC Purification
Preparative purification of peptides was conducted on a SHIMADZU LC-20AR system using a Daisogel C18 preparative column (20 × 250 mm) at a flow rate of 10 mL/min.
Solvent systems:
Buffer A: acetonitrile + 0.1% TFA
Buffer B: water + 0.1% TFA
Gradient for purification:
Linear increase of Buffer A from 10% → 90% over 50 min.
Purified peptides were collected, lyophilized, and characterized by ESI-Q-TOF-MS.
Reference:
Yinxue Fu, Chunhui Dou, Xiaoyang Gao, Shanping Ji, Xuemei Zhao, Jingwen Xue, Hao Yang, Nannan Song, Chunyu Zhang, Changlong Wang, and Yulei Li. Journal of Medicinal Chemistry 2025 68 (19), 20435-20448
DOI: 10.1021/acs.jmedchem.5c01550
